| Material Testing:
The purpose of this experiment is to find the potential difference between
different materials in a galvanic couple. Different conditions for the
experiment will provide information to reach the best conditions for the
greater potential difference of a galvanic couple. These conditions will
include distance between the metals and material surface area ratio for
each of the galvanic couples.
Short Term Testing
Four tests were conducted for the short term testing. These test included:
open circuit test varying the ratio between the anode and cathode, open
circuit test varying the distance between the anode and cathode material,
a closed circuit at 12” apart and 4” apart, and a corrosion
rate test. Theses tests were conducted for the galvanic couple of titanium
and aluminum, and for the galvanic couple of stainless steel 304 and aluminum.
The first test conducted varied the ratio between the anode and cathode
material. The cathodes to anode surface area ratio (R) used were: 5:3,
1, 2:3, and 1:3. The following graph shows the data collected for the
stainless steel and aluminum couple.
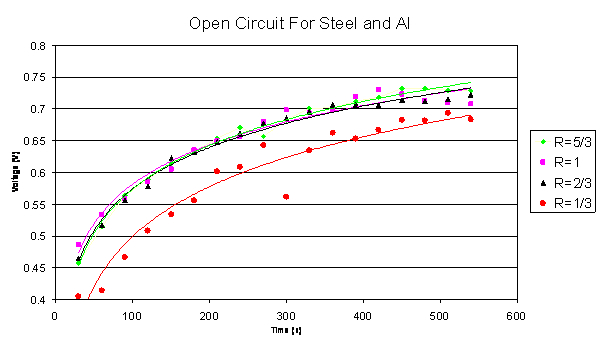
Open Circuit Graph 1
The data plotted in this graph indicates that the larger
the cathode material is compared to the anode material, the larger the
voltage output. This trend is the same for the galvanic couple of titanium
and aluminum. Below is the open circuit graph for aluminum and titanium.
The same ratios were used.
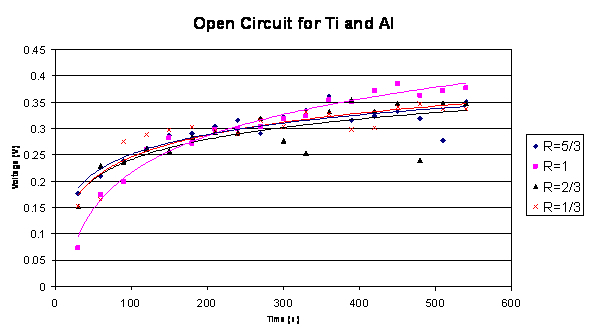
Open Circuit Graph 2
The voltage for the galvanic couple as shown in Graph 2
is lower than the voltage shown in Graph 1. The need for this design requires
the materials chosen to produce the greatest potential. Therefore, the
following information will focus in on the data collected for the stainless
steel 304 and aluminum.
The second test performed was an open circuit test that varied the distance
between the cathode material stainless steel 304 and the anode material
aluminum. The distance (D) varied between 4”, 8”, and 12”.
Below this information is plotted in Graph 3.
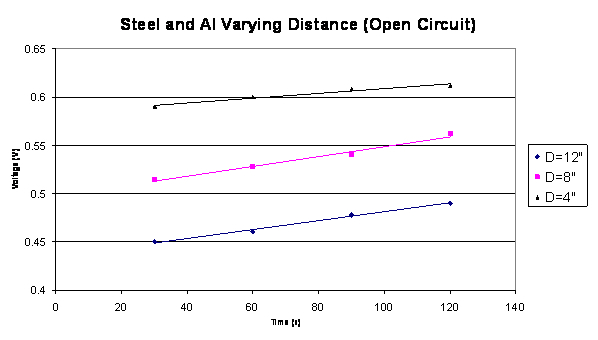
Varying Distance Graph 3
The data plotted shows that the smaller the distance between
the cathode and anode material, the larger the voltage output. This concludes
that the materials in the couple have to be close together without touching
in order to avoid short circuiting. The optimal distance between the cathode
and anode was experimentally determined to be 1” apart.
The third test performed was the closed circuit test. The closed circuit
used a 1 Ohm resistor. This test varied the ratio (R) between the anode
and cathode for when the materials were 12” apart and when they
were 4” apart. Based off the information from Graph 3, the data
from when the materials are only 4” apart is relevant. Below is
the graph for the closed circuit for stainless steel 304 and aluminum.
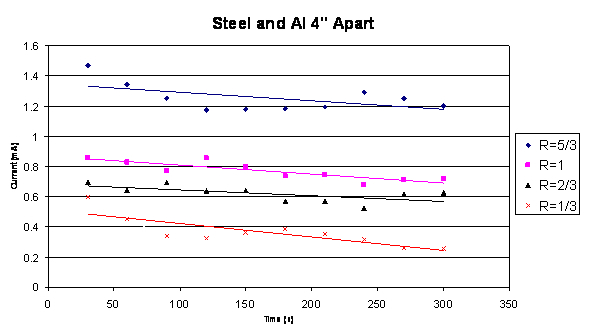
Closed Circuit Graph 4
The data plotted in Graph 4 shows the relationship between
the ratio (R) between the cathode and anode material and the current.
The larger the cathode is in comparison to the anode, the larger the current
and the larger the current the more power that can be produced.
The fourth test conducted was the corrosion rate test. The mass of the
materials were weighed in the beginning and then at the end of 3 days
the mass was weighed again. This data is shown in the graph below.
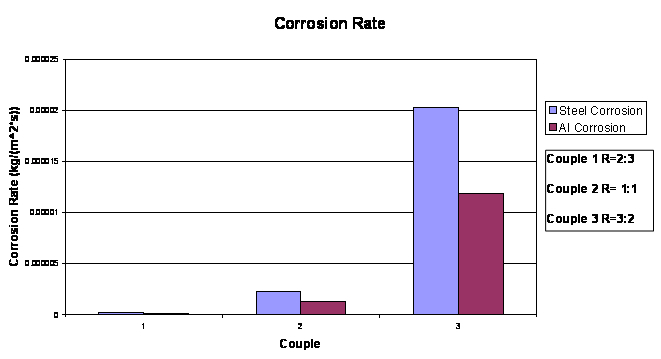
Corrosion Rate
The corrosion rate for the materials used, Steel and Aluminum,
is shown for three different couples 1, 2, and 3, where the cathode to
anode surface area ratio were 2/3, 1 and 3/2 respectively.
Long Term Testing
The long term data was obtained using the data acquisition system. Four
different couples were tested. Couples 1 and 2 were Titanium and Aluminum
at a cathode to anode surface area ratio of 5/3 and 1 respectively. Couples
3 and 4 were Steel and Aluminum at a cathode to anode surface area ratio
of 5/3 and 1 respectively. This data is presented in the following graph.
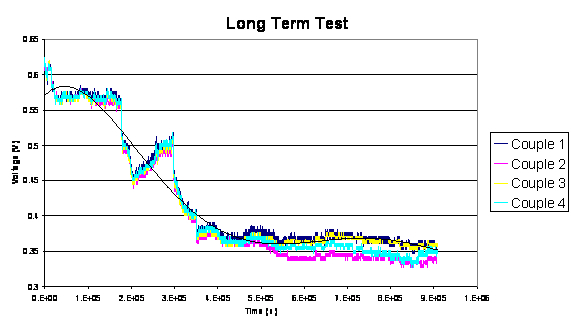
Long Term Experiment
The data plotted in this graph shows that couple one produces
the largest voltage output. Couple one is titanium and aluminum which
slightly has the greatest voltage output over the entire time. This information
is not coherent with the short term data, but that is due to the fact
that the long term data is more precise.
The following two graphs show the long term data collected
from two different couples using magnesium as the anode and steel and
titanium as cathodes respectively.
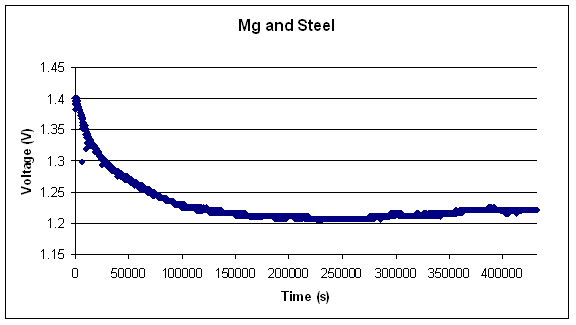
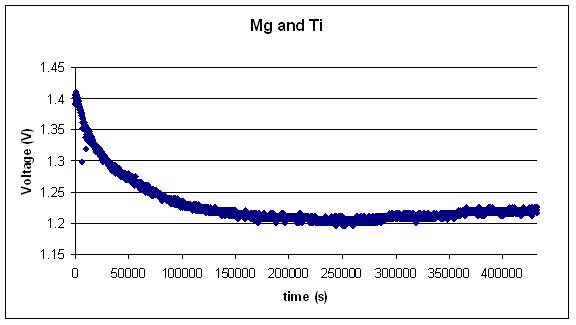
Experimental Conclusions:
The data collected from the different experiments conclude three key
aspects. These include the material combination for the galvanic couple,
the ratio of the surface area between the cathode and the anode, and the
distance between the anode and cathode.
The galvanic couples must consist of a Steel or Titanium plate as the
cathode and Magnesium as the anode material. This will ensure a higher
and steadier potential than the other readily available materials for
a longer period of time.
After analyzing the corrosion graph it was concluded that a higher material
surface ratio will ensure a higher potential difference. But at the same
time this ratio will speed up the corrosion process; for this reason a
surface ratio of 1 must be used in order to have a high potential difference
and an effective life span.
The distance between the cathode and anode has to be no farther than
1” apart to maximize the voltage output. Graph 3 supports this conclusion
by showing as the distance between the cathode and anode gets smaller
the greater the voltage output.
|

