|
|
|
|
|
Next: 7.3 Conservation Laws and Symmetries |
|
The time evolution of systems may be found using the Schrödinger equation as described in the previous section. However, that requires the energy eigenfunctions to be found. That might not be easy.
For some systems, especially for macroscopic ones, it may be sufficient to figure out the evolution of the expectation values. An expectation value of a physical quantity is the average of the possible values of that quantity, chapter 4.4. This section will show how expectation values may often be found without finding the energy eigenfunctions. Some applications will be indicated.
The Schrödinger equation requires that the expectation value ![]()
![]()
![]()
The above evolution equation for expectation values does not require the energy eigenfunctions, but it does require the commutator.
Note from (7.4) that if an operator ![]()
![]()
![]()
![]()
One application of equation (7.4) is the so-called “virial theorem” that relates the expectation potential and kinetic energies of energy eigenstates, {A.17}. For example, it shows that harmonic oscillator states have equal potential and kinetic energies. And that for hydrogen states, the potential energy is minus two times the kinetic energy.
Two other important applications are discussed in the next two subsections.
Key Points
- A relatively simple equation that describes the time evolution of expectation values of physical quantities exists. It is fully in terms of expectation values.
- Variables which commute with the Hamiltonian have the same time-independent statistics as energy.
- The virial theorem relates the expectation kinetic and potential energies for important systems.
The purpose of this section is to show that even though Newton's equations do not apply to very small systems, they are correct for macroscopic systems.
The trick is to note that for a macroscopic particle, the position and
momentum are very precisely defined. Many unavoidable physical
effects, such as incident light, colliding air atoms, earlier history,
etcetera, will narrow down position and momentum of a macroscopic
particle to great accuracy. Heisenberg's uncertainty relationship
says that they must have uncertainties big enough that
![]()
![]()
![]() ,
,![]()
With little uncertainty in position and momentum, both can be approximated accurately by their expectation values. So the evolution of macroscopic systems can be obtained from the evolution equation (7.4) for expectation values given in the previous subsection. Just work out the commutator that appears in it.
Consider one-dimensional motion of a particle in a potential ![]()
![]()
Now according to evolution equation (7.4), the
expectation position ![]()
![\begin{displaymath}
\frac{{\rm d}\langle x \rangle}{{\rm d}t}
= \left\langle \...
...{\widehat p}_x^2}{2m} + V(x),{\widehat x}\right] \right\rangle
\end{displaymath}](img1580.gif) |
(7.5) |
So the rate of change of expectation position becomes:
| (7.6) |
To figure out how the expectation value of momentum varies, the
commutator ![]()
![]()
![]() ,
,![]() .
.![]()
![]()
![]()
![]()
![]() .
.
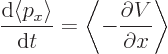 |
(7.7) |
This is Newton's second law in terms of expectation values: Newtonian mechanics defines the negative derivative of the potential energy to be the force, so the right hand side is the expectation value of the force. The left hand side is equivalent to mass times acceleration.
The fact that the expectation values satisfy the Newtonian equations is known as “Ehrenfest's theorem.”
For a quantum-scale system, however, it should be cautioned that even the expectation values do not truly satisfy Newtonian equations. Newtonian equations use the force at the expectation value of position, instead of the expectation value of the force. If the force varies nonlinearly over the range of possible positions, it makes a difference.
There is a alternative formulation of quantum mechanics due to Heisenberg that is like the Ehrenfest theorem on steroids, {A.12}. Here the operators satisfy the Newtonian equations.
Key Points
- Newtonian physics is an approximate version of quantum mechanics for macroscopic systems.
- The equations of Newtonian physics apply to expectation values.
The Heisenberg uncertainty relationship provides an intuitive way to
understand the various weird features of quantum mechanics. The
relationship says ![]()
![]()
![]() ,
,![]()
![]()
Now special relativity considers the energy ![]()
![]()
![]()
There is a difference, however. In Heisenberg’s original
relationship, the uncertainties in momentum and positions are
mathematically well defined. In particular, they are the standard
deviations in the measurable values of these quantities. The
uncertainty in energy in the energy-time uncertainty relationship can
be defined similarly. The problem is what to make of that
uncertainty in time
![]() .
.
One way to address the problem is to look at the typical evolution time of the expectation values of quantities of interest. Using careful analytical arguments along those lines, Mandelshtam and Tamm succeeded in giving a meaningful definition of the uncertainty in time, {A.18}. Unfortunately, its usefulness is limited.
Ignore it. Careful analytical arguments are for wimps! Take out your
pen and cross out
Write in
![]() .
.any time difference you want.
Cross out
and write in “any energy difference
you want.” As long as you are at it anyway, also cross out
![]()
This can be
justified because both are mathematical symbols. And inequalities are
so vague anyway. You have now obtained the popular version of the
Heisenberg energy-time uncertainty equality:
![]() ”
”![]() .
.
This is an extremely powerful equation that can explain anything in quantum physics involving any two quantities that have dimensions of energy and time. Be sure, however, to only publicize the cases in which it gives the right answer.
Key Points
- The energy-time uncertainty relationship is a generalization of the Heisenberg uncertainty relationship. It relates uncertainty in energy to uncertainty in time. What uncertainty in time means is not obvious.
- If you are not a wimp, the answer to that problem is easy.