|
|
|
|
|
Next: 3.2 The Heisenberg Uncertainty Principle |
|
This section describes the view quantum mechanics has of nature, which is in terms of a mysterious function called the “wave function”.
According to quantum mechanics, the way that the old Newtonian physics
describes nature is wrong if examined closely enough. Not just a bit
wrong. Totally wrong. For example, the Newtonian picture for a particle of mass ![]()
The problems? A numerical position for the particle simply does not exist. A numerical velocity or linear momentum for the particle does not exist.
What does exist according to quantum mechanics is the so-called wave
function ![]() .
.![]() ,
,
The physical meaning of the wave function is known as “Born's statistical interpretation”: darker regions are regions
where the particle is more likely to be found if the location is
narrowed down. More precisely, if ![]()
![]()
![]()
| (3.1) |
(And if such a position measurement is actually done, it affects the wave function: after the measurement, the new wave function will be restricted to the volume to which the position was narrowed down. But it will spread out again in time if allowed to do so afterwards.)
The particle must be found somewhere if you look everywhere. In
quantum mechanics, that is expressed by the fact that the total
probability to find the particle, integrated over all possible
locations, must be 100% (certainty):
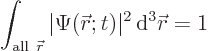 |
(3.2) |
The position of macroscopic particles is typically very much narrowed
down by incident light, surrounding objects, earlier history,
etcetera. For such particles, the blob size
of the
wave function is extremely small. As a result, claiming that a
macroscopic particle, is, say, at the center point of the wave
function blob may be just fine in practical applications. But when you
are interested in what happens on very small scales, the nonzero blob
size can make a big difference.
In addition, even on macroscopic scales, position can be ill defined.
Consider what happens if you take the wave function blob apart and
send half to Mars and half to Venus. Quantum mechanics allows it;
this is what happens in a scattering
experiment.
You would presumably need to be extremely careful to do it on such a
large scale, but there is no fundamental theoretical objection
in quantum mechanics. So, where is the particle now? Hiding on Mars?
Hiding on Venus?
Orthodox quantum mechanics says: neither. It will pop up on one of the two planets if measurements force it to reveal its presence. But until that moment, it is just as ready to pop up on Mars as on Venus, at an instant's notice. If it was hiding on Mars, it could not possibly pop up on Venus on an instant's notice; the fastest it would be allowed to move is at the speed of light. Worse, when the electron does pop up on Mars, it must communicate that fact instantaneously to Venus to prevent itself from also popping up there. That requires that quantum mechanics internally communicates at speeds faster than the speed of light. That is called the Einstein-Podolski-Rosen paradox. A famous theorem by John Bell in 1964 implies that nature really does communicate instantaneously; it is not just some unknown deficiency in the theory of quantum mechanics, chapter 8.2.
Of course, quantum mechanics is largely a matter of inference. The
wave function cannot be directly observed. But that is not as strong
an argument against quantum mechanics as it may seem. The more you
learn about quantum mechanics, the more its weirdness will probably
become inescapable. After almost a century, quantum mechanics is
still standing, with no real more reasonable
competitors, ones that stay closer to the common sense picture. And
the best minds in physics have tried.
From a more practical point of view, you might object that the Born
interpretation cheats: it only explains what the absolute value of the
wave function is, not what the wave function itself is. And you would
have a very good point there. Ahem. The wave function ![]()
![]() .
.
However, for unknown reasons nature always computes
with a wave function, never with probabilities. The classical example
is where you shoot electrons at random at a tiny pinhole in a wall.
Open up a second hole, and you would expect that every point behind
the wall would receive more electrons, with another hole open. The
probability of getting the electron from the second hole should add to
the probability of getting it from the first one. But that is not
what happens. Some points may now get no electrons at all. The wave
function trace passing through the second hole may arrive with the
opposite sign of the wave function trace passing through the first
hole. If that happens, the net wave function becomes zero, and so its
square magnitude, the probability of finding an electron, does too.
Electrons are prevented from reaching the location by giving them an
additional way to get there. It is weird. Then again, there is
little profit in worrying about it.
Key Points
- According to quantum mechanics, particles do not have precise values of position or velocity when examined closely enough.
- What they do have is a “wave function“ that depends on position.
- Larger values of the magnitude of the wave function, (indicated in this book by darker regions,) correspond to regions where the particle is more likely to be found if a location measurement is done.
- Such a measurement changes the wave function; the measurement itself creates the reduced uncertainty in position that exists immediately after the measurement.
- In other words, the wave function is all there is; you cannot identify a hidden position in a given wave function, just create a new wave function that more precisely locates the particle.
- The creation of such a more localized wave function during a position measurement is governed by laws of chance: the more localized wave function is more likely to end up in regions where the initial wave function had a larger magnitude.
- Proper wave functions are normalized.