|
|
|
|
Specific heat and work:
Continuity (mass conservation):
The first law of thermo (energy conservation):
Work:
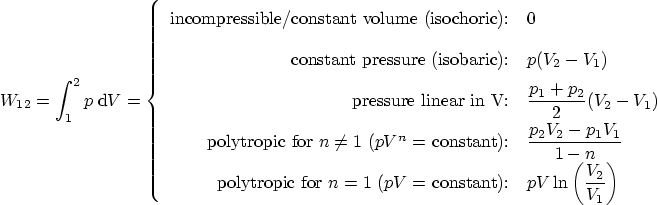
No heat is added or removed if the process is adiabatic.
Exams 3 and final only
More generally:

The second law for reversible adiabatic (isentropic) processes:
The second law of thermo for general processes:
The first law as a rate equation:
Work as a rate equation:
For an ideal gas:
For liquids and solids:
The control volume is assumed to be steady state in all formulae below.
Specific work output and heat added (i.e., per unit mass flowing through):
In- and outflow velocities and pipe cross-sectional areas:
Continuity (mass conservation):
The first law of thermo (energy conservation):
Exams 3 and final only
The second law of thermo for a reversible adiabatic (isentropic)
process inside a single entrance and exit CV:
The second law of thermo for a reversible isothermal process inside a
single entrance and exit CV:
The second law for a general control volume:
Specific work done during a reversible process inside a single
entrance and exit CV:
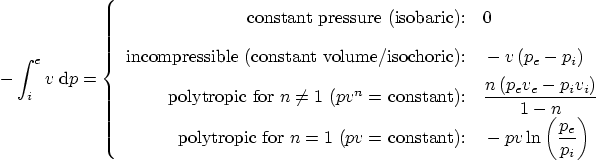
Special case of (a) ideal gas, (2) polytropic, ![]() , (including
isentropic constant specific heats, then
, (including
isentropic constant specific heats, then ![]() ,) and (3) reversible:
,) and (3) reversible:
![\begin{displaymath}
- \int_i^e v \; {\rm d} p =
\frac{nRT_1}{1-n}\left[\left(\frac{P_2}{P_1}\right)^{(n-1)/n}-1\right]
\end{displaymath}](_img88.gif)
Special case of (a) ideal gas, (2) constant specific heats, (3) polytropic,
![]() (isothermal). and (4) reversible:
(isothermal). and (4) reversible:
Adiabatic turbine and compressor/pump efficiencies:
End exams 3 and final only