Specific heat and work:
Continuity (mass conservation):
The first law of thermo (energy conservation):
Work:
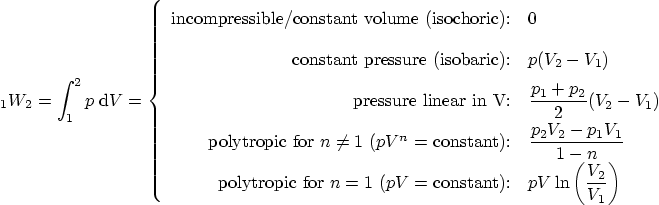
Heat added:
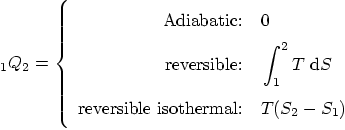
The second law for reversible adiabatic (isentropic) processes:
The second law of thermo for general processes:
The first law as a rate equation:
Work as a rate equation:
For an ideal gas:
For liquids and solids:
The control volume is assumed to be steady state in all formulae below.
Specific work output and heat added (i.e., per unit mass flowing through):
In- and outflow velocities and pipe cross-sectional areas:
Continuity (mass conservation):
The first law of thermo (energy conservation):
The second law of thermo for a reversible adiabatic (isentropic)
process inside a single entrance and exit CV:
The second law of thermo for a reversible isothermal process inside a
single entrance and exit CV:
The second law for a general control volume:
Specific work done during a reversible process inside a single
entrance and exit CV:
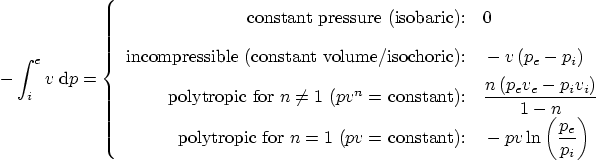
Adiabatic turbine and compressor/pump efficiencies: