| Quantum Mechanics Solution Manual |
|
© Leon van Dommelen |
|
4.3.3.4 Solution hydc-d
Question:
What is the color of the light emitted in a Balmer transition from an energy level  with a high value of
with a high value of 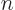 to
to 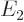 ?
?
Answer:
It is like the previous question, except  will be approximately zero at high values of
will be approximately zero at high values of 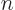 .
.
Dividing by 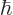
 1.054 10
1.054 10 J s gives the angular frequency to be 5.17 10
J s gives the angular frequency to be 5.17 10 /s, and then the wave length is 364 nm. That will be near ultraviolet. The transition from
/s, and then the wave length is 364 nm. That will be near ultraviolet. The transition from  will produce blue-green light, from
will produce blue-green light, from  indigo, and from
indigo, and from  violet.
violet.
![]()
![]()
![]() ?
?![]()
![]() .
.