| This page contains notes on Heat (Q) and derivations for Work (1W2) equations. |
|
| Heat (Q) |
energy transferred across the system boundary due to a temperature difference.
Heat is transferred from the system at higher temperature to the one at lower temperature.
Heat is an inexact differential -- it is path dependent.
| Units |
| SI | J, joule |
| English | Btu, British Thermal Unit
1 Btu = 778.17 ft-lbf
1 Btu = heat required to raise 1 lb water from 59.5oF to 60.5oF.
[ 1 calorie = heat required to raise 1 gm water from 14.5oC to 15.5oC. ] |
The rate of heat transfer to/from system
| Q-dot = | 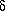 Q Q
dt |
Specific heat transfer,
- Heat transferred TO a system is POSITIVE.
- Heat transferred FROM a system is NEGATIVE.
Remember,
- Work done BY a system is POSITIVE.
- Work done ON a system is NEGATIVE.
|
|
| Some comments on WORK and HEAT |
|
- Both are transient
- Both are boundary phenomena
- Both are path functions
|
| Links to derivations for Work (1W2) equations.
|
| W for a process where pV=constant
|
| W for a process where pVn=constant
| 1W2 = |
p2V2 - p1V1
--------------------
1 - n |
|
| W for a process expanding against a LINEAR spring
| 1W2 = |
(1/2)(p1 + p2)(V2 - V1) = p1(V2 - V1) + (1/2)(p2 - p1)(V2 - V1) |
|
|
| - END |
 Q
Q