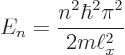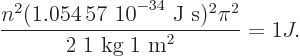| Quantum Mechanics Solution Manual |
|
© Leon van Dommelen |
|
3.5.6.3 Solution pipee-c
Question:
What is the eigenfunction number, or quantum number, 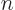 that produces a macroscopic amount of energy, 1 J, for macroscopic values
that produces a macroscopic amount of energy, 1 J, for macroscopic values 
 1 kg and
1 kg and 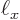
 1 m? With that many energy levels involved, would you see the difference between successive ones?
1 m? With that many energy levels involved, would you see the difference between successive ones?
Answer:
Putting the generic expression for the eigenvalues,
equal to 1 J and plugging in the given numbers:
Solving for 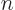 , you get
, you get 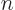
 4.268 64 10
4.268 64 10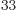 . Obviously, there is no way to distinguish that many energy levels. A calculator cannot even display all 34 digits of this number, even if you knew
. Obviously, there is no way to distinguish that many energy levels. A calculator cannot even display all 34 digits of this number, even if you knew 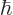 to enough digits accuracy to compute 34 digits.
to enough digits accuracy to compute 34 digits.
![]()
![]()
![]()
![]()
![]()