Research and Development on Some Critical Issues for High Energy and Power Densities, and Good Lifespan of Li-air Batteries
Project Objective: We propose some fundamental researches and material developments targeting some crucial issues in order to improve the energy and power densities, and lifespan of Li-air batteries. Our research objectives include (1) development of polymer membranes which has high Li ion conductivity and low interface resistance with solution electrolytes; (2) development of air electrodes with desired catalytic functions for promoting O2 reaction during the discharge; and (3) design and stimulation of air electrode structure using a in-house simulation software for optimizing the energy and power densities of Li-air batteries.
From previous our research during past years, we have build a strong Li-air battery research group at Florida State University with multidisciplinary expertise in battery electrode material and construction, solid electrolyte development, battery device assembly and characterization, and battery modeling and stimulation. We have also achieved some significant results in Li-air battery research and development as follows:
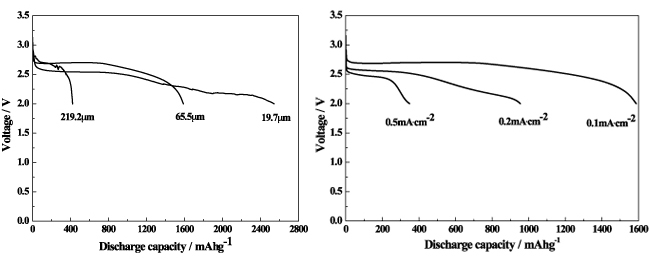
We have developed a model for predication the gravimetric and volumetric energy densities of Li-air batteries using non-aqueous/aqueous hybrid electrolytes.[1,2] The theoretical gravimetric/volumetric capacities and energy densities are calculated based on the minimum weight of the electrolyte and volume of air electrode needed for completion of the electrochemical reaction with Li metal as anode electrode. A physics-based model is proposed for the simulation of Li-air batteries. The model is carefully calibrated against published data and is used to simulate standard Li-air batteries with nonaqueous (organic) electrolyte.[3] The model can calculate number important parameters including the potentials of Li ion and electron, concentrations of O2 and Li ion, porosity, electrochemical reaction rate, and so on as functions of location inside the Li-air cells and the state-of-the-charge (SOC). Li-air cells based on Li foil as anode electrode, free-standing carbon nanotube/nanofiber mixed buckypaper as air (cathode) electrode, and organic electrolyte were assembled. The air electrode was made with single-wall carbon nanotube and carbon nanofiber without any binder. It was found that the discharge capacity was strongly dependent on both the discharge current density and the thickness of the air electrode as shown in Fig 1 and 2.[4]
Fig. 1 Discharge curves from Li-air cells (a) made with different thicknesses under a constant current density of 0.1 mA/cm2 and discharged at constant current densities of 0.1, 0.2 and 0.5 mA/cm2.
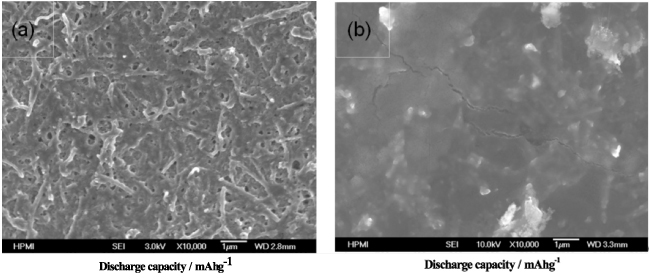
Fig. 2 SEM images of the air electrode surfaces at (a) separator and (b) air sides after discharge at 0.1 mA/cm2.
References
- J.P. Zheng, R.Y. Liang, M. Hendrickson, and E.J. Plichta, "The Theoretical Energy Density of Li-Air Batteries", J. Electrochem. Soc. 155, A432 (2008).
- J.P. Zheng, P. Andrei, M. Hendrickson, and E.J. Plichta, "The Energy Densities of Rechargeable Li-air and Li-air Flow Batteries", J. Electrochem. Soc. 158, A43 (2011).
- P. Andrei, J.P. Zheng, M. Hendrickson, and E.J. Plichta, "Some possible approaches for improving the energy density of Li-air batteries" J. Electrochem. Soc. 157, A953 (2010).
- G.Q. Zhang, R.Y. Liang, J.P. Zheng, M. Hendrickson, and E.J. Plichta, "Lithium-air Batteries Using SWNT/CNF Buckypapers as Air Electrodes" J. Electrochem. Soc. 157, A953 (2010).
Contact
- Jim P. Zheng, Professor of Electrical Engineering
- Email: zheng@eng.fsu.edu | Phone: (850) 410-6464
Sponsor
- US Army Communications-Electronics Research, Development, and Engineering Center